Study on the Synthesis and Performance of a Large Temperature Difference Retarder for Oil Well Cement(Part 1)
Abstract
In response to the problem of excessive or non setting of the top cement slurry caused by the large temperature difference between the top and bottom of the cement slurry in the long cementing section, this article synthesized a high temperature difference retarder PADBrC for oil well cement using 2-methyl-2-acrylamidopropane sulfonic acid (AMPS), acrylic acid (AA), methacryloxyethyl trimethylammonium chloride (DMC), and long-chain quaternary ammonium salt (DBrC) as monomers. Its structure and properties were characterized by infrared spectroscopy, nuclear magnetic resonance hydrogen spectroscopy, thermogravimetric analysis, and scanning electron microscopy. The results show that PADBrC inhibits the hydration of cement by inhibiting the formation of Ca (OH)2 and hydrated calcium silicate gel (C-S-H) in cement; PADBrC can maintain good thickening performance of cement slurry under high temperature conditions of 150℃, with a thickening time of 235 minutes and a stable thickening curve; PADBrC can increase the compressive strength of cement paste cured under low temperature conditions of 60℃ for 24 hours to more than 14MPa. Therefore, PADBrC has good temperature difference adaptability and can meet the requirements of large temperature difference cementing construction at 60-150℃.
With the increasing demand for oil and gas resources by the country, major oil fields are increasingly deepening their exploration and development work towards deep formations. The number of deep and ultra deep wells is also gradually increasing, and the geological environment they face is becoming increasingly complex. To ensure the safety of high-temperature deep well cementing construction, a retarder needs to be added to the cement slurry. Its main function is to extend the cement hydration induction period, improve the rheological properties of the cement slurry under high-temperature cementing conditions, and meet the pumping time requirements underground. However, as the depth of the well continues to increase, the sealing section becomes longer and longer, resulting in a larger temperature difference between the bottom and wellhead. When the cement slurry returns from the high-temperature and high-pressure conditions at the bottom to the low-temperature section at the top, the large temperature difference between the upper and lower parts causes the use of the oil well cement retarder across temperature sections, resulting in phenomena such as super retarding or non setting of the top cement slurry, which poses a huge challenge to the safety of cementing construction.
Common retarders include hydroxycarboxylic acids, cellulose and its derivatives, lignosulfonate and its derivatives, carbohydrate compounds, inorganic acids, and organic phosphate retarders. These retarders are cheap and easy to obtain, but most of them have a narrow temperature range, resulting in poor retarder effect at high temperatures and slow development of cement strength at low temperatures. At present, due to the controllability of polymer synthesis, different functional monomers can be polymerized together, and molecular chain length and size can be adjusted through molecular design to obtain retarders with good comprehensive performance and meet engineering requirements. This process has become a hot research topic for domestic surgical researchers.
This article adopts the solution polymerization in-situ intercalation method, using 2-methyl-2-acrylamidopropane sulfonic acid (AMPS), acrylic acid (AA), and methacryloxyethyl trimethylammonium chloride (DMC) as polymerization monomers. By adjusting the reaction conditions, an organic-inorganic composite high-temperature resistant retarder HTR-5 is prepared. Then, a quaternary ammonium salt monomer DBrC with long hydrophobic side chains is introduced, and an oil well cement large temperature difference retarder PADBrC is synthesized through aqueous solution free radical polymerization. This effectively solves the problem of top cement slurry super-retardation or non setting caused by the long sealing section in well cementing.
1. Experimental Section
1.1 Instruments and Reagents
WQF520 infrared spectrometer; Bruker AVANCE III HD 400 nuclear magnetic resonance spectrometer; Alliance e2695 gel chromatograph; Labsys EVO thermogravimetric analyzer; Quanta 250 FEG environmental scanning electron microscope; TG-8040B high-temperature and high-pressure thickener; NYL-300 pressure testing machine.
AMPS (Industrial Pure, Qingdao Aolu Petroleum Machinery Co., Ltd.); AA (Analytical Pure, Chengdu Kelong Chemical Reagent Factory); DMC (Analytical Pure, Shanghai Aladdin Biochemical Technology Co., Ltd.); Dimethylaminoethyl methacrylate (DMAEMA, analytical pure, Shanghai Titan Technology Co., Ltd.); Acetone, potassium persulfate, ether, sodium hydroxide (all analytical grade, Chengdu Kelong Chemical Reagent Factory); Potassium bromide (spectral purity, Chengdu Kelong Chemical Reagent Factory); G-grade oil well cement and quartz sand (both industrial products, Jiahua Special Cement Co., Ltd.); Fluid loss agent SWJ-1 and drag reducer SWJZ-1 (both industrial grade, Shengli Oilfield); Defoamer (PC-X60L, industrial grade, CNOOC).
1.2 Synthesis
Mix 3.15g of DMAEMA with 30mL of acetone and add to a three necked flask. Heat and stir to 55℃. Slowly add 3.41g of bromochexane dropwise into the mixed solution and react for 12 hours to obtain a colorless solution. After cooling, obtain a white precipitate. Pour the precipitate into ether and wash repeatedly. After vacuum drying, obtain the quaternary ammonium monomer DBrC. Add 18.88g of AMPS, 4.93g of AA, 0.29g of DMC, and 0.02g of DBrC to the beaker for dissolution, and then mix the resulting solution evenly. Adjust the pH to 6 with a mass fraction of 10.0% NaOH solution, and heat the solution with nitrogen to 60℃. After stabilizing the temperature, a 0.2% mass fraction potassium persulfate initiator was added, and the reaction lasted for 6 hours to obtain a transparent and viscous liquid. The liquid was poured into acetone and washed repeatedly. After vacuum drying, it was crushed to obtain the final product PADBrC.
1.3 Performance Testing Methods
According to the relevant provisions of GB/T 19139-2012 "Test Methods for Oil Well Cement", the cement slurry is prepared. The 60℃ and 90℃ cement slurry system formula is: Jiahua G-grade cement, 600.00g+PAD-BrC, 12.00g+fluid loss agent SWJ-1, 6.00g+drag reducer SYJ-1, 1.80g+defoamer PC-X60L, 0.50g, water cement ratio 0.44.
The formula of the 120℃ and 150℃ cement slurry system is: Jiahua G-grade cement, 600.00g+quartz sand, 210.00g+PADBrC, 16.20g+fluid loss agent SWJ-1, 8.10g+drag reducer SYJ-1, 2.40g+defoamer PC-X60L, 0.50g, water cement ratio 0.56.
Evaluate the thickening performance of cement slurry at high temperature (60-150℃) and the compressive strength of cement stone cured at low temperature (60-120℃) for 24 hours using the reference method.
2. Results and Discussion
2.1 Characterization
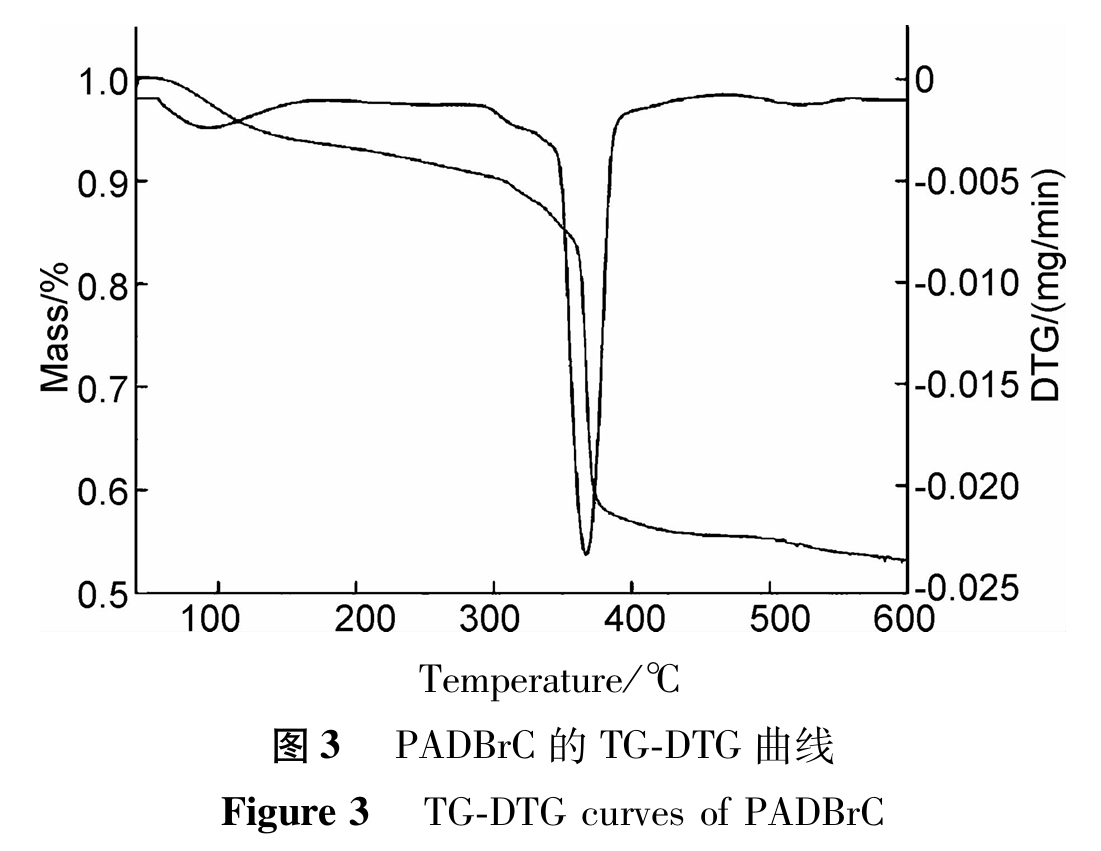
(1). IR analysis
The infrared spectrum of PADBrC is shown in Figure 1. As shown in Figure 1, 3478cm-1 is the stretching vibration absorption peak of -OH, 1671cm-1 is the stretching vibration absorption peak of -C=O, 1226cm-1 and 1043cm-1 are the stretching vibration absorption peaks of -SO3H, 2931cm-1 is the characteristic absorption peak of -CH2-, 1307cm-1 is the C-N stretching vibration absorption peak of quaternary ammonium salt, and 1110cm-1 is the asymmetric stretching vibration peak of C-O-C in ester. There is no vibration absorption peak of C=C (1670cm-1) in the spectrum, indicating that the purified copolymer does not contain monomers that did not participate in the reaction. From the above analysis, it can be seen that the characteristic absorption bands of the functional groups corresponding to the four monomers appear in the spectrum, indicating that all four monomers are involved in the chemical reaction.
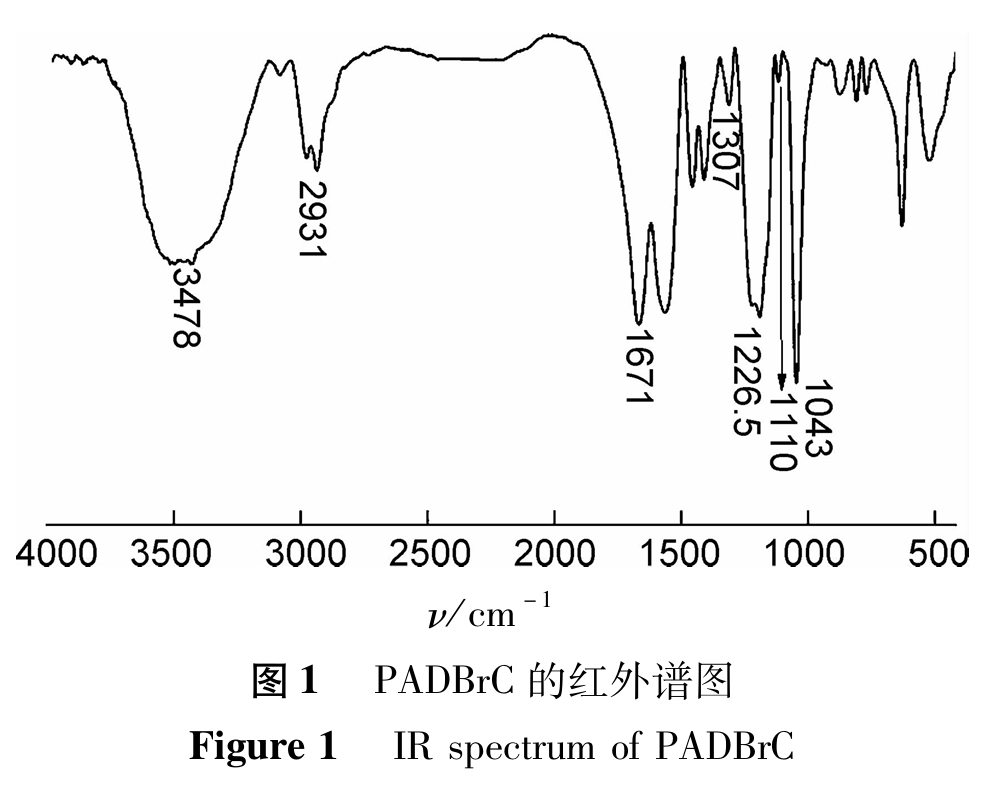
(2). 1H NMR analysis
The nuclear magnetic resonance hydrogen spectrum of PADBrC is shown in Figure 2. As shown in Figure 2, δ 1.46 and δ 2.03 is the proton peak of -CH2- and -CH- in the main chain, δ 3.14 is the proton peak of -CH2- connected to -SO3H in AMPS, δ 3.32 is the proton peak of -CH2- in [N+(CH3) 3] connected to quaternary ammonium salts, δ 3.51- δ 3.57 is the proton peak of -CH3- in quaternary ammonium salts. There is no proton peak of H connected to the olefin in the spectrum, indicating that there are no unpolymerized monomers in the monomer, indicating that the obtained product is the target product.
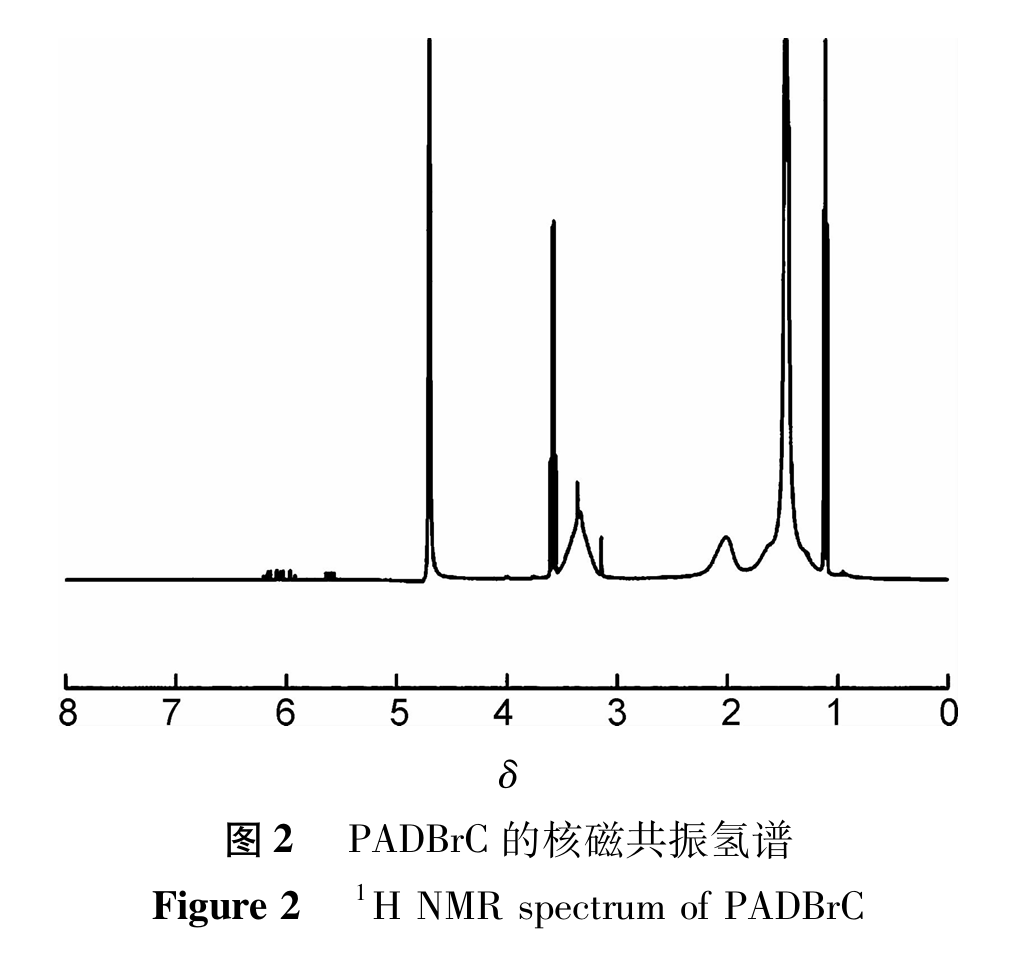
(3). TG-DTG analysis
The TG-DTG curve of PADBrC is shown in Figure 3. From Figure 3, it can be seen that within the temperature range of 50℃ to 600℃, there are a total of three weight loss regions in the thermogravimetric curve of PADBrC. The first weight loss area is between 58-150℃, with a weight loss rate of about 6.49%. This is due to the evaporation of free water and bound water in polymer PADBrC as the temperature increases; The second weight loss region is between 209 and 450℃, with a weight loss rate of 36.02%. The corresponding DTG curve shows a peak, with a peak temperature of 359℃. This phenomenon is due to the breakage of the main chain in the polymer; The third weight loss region is between 470-570℃, with a weight loss rate of approximately 2.30%, which is due to molecular chain carbonization. In summary, when the temperature is higher than 359℃, the polymer shows significant mass loss, indicating that PADBrC has excellent thermal stability and can withstand high temperatures of 359℃.
(4). Molecular weight
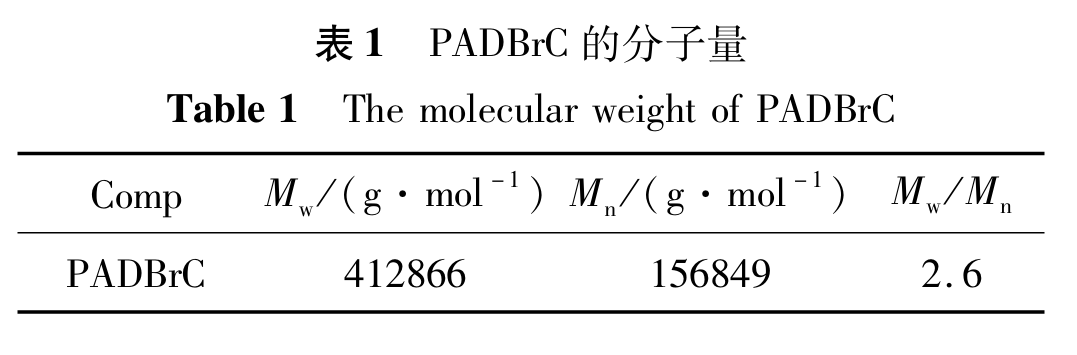
The molecular weight of PADBrC was tested by gel permeation chromatography. According to Table 1, the weight average molecular weight (Mw) of PADBrC was 412866, the number average molecular weight (Mn) was 156849, and the molecular weight distribution index (Mw/Mn) was 2.6, indicating a wide distribution index.
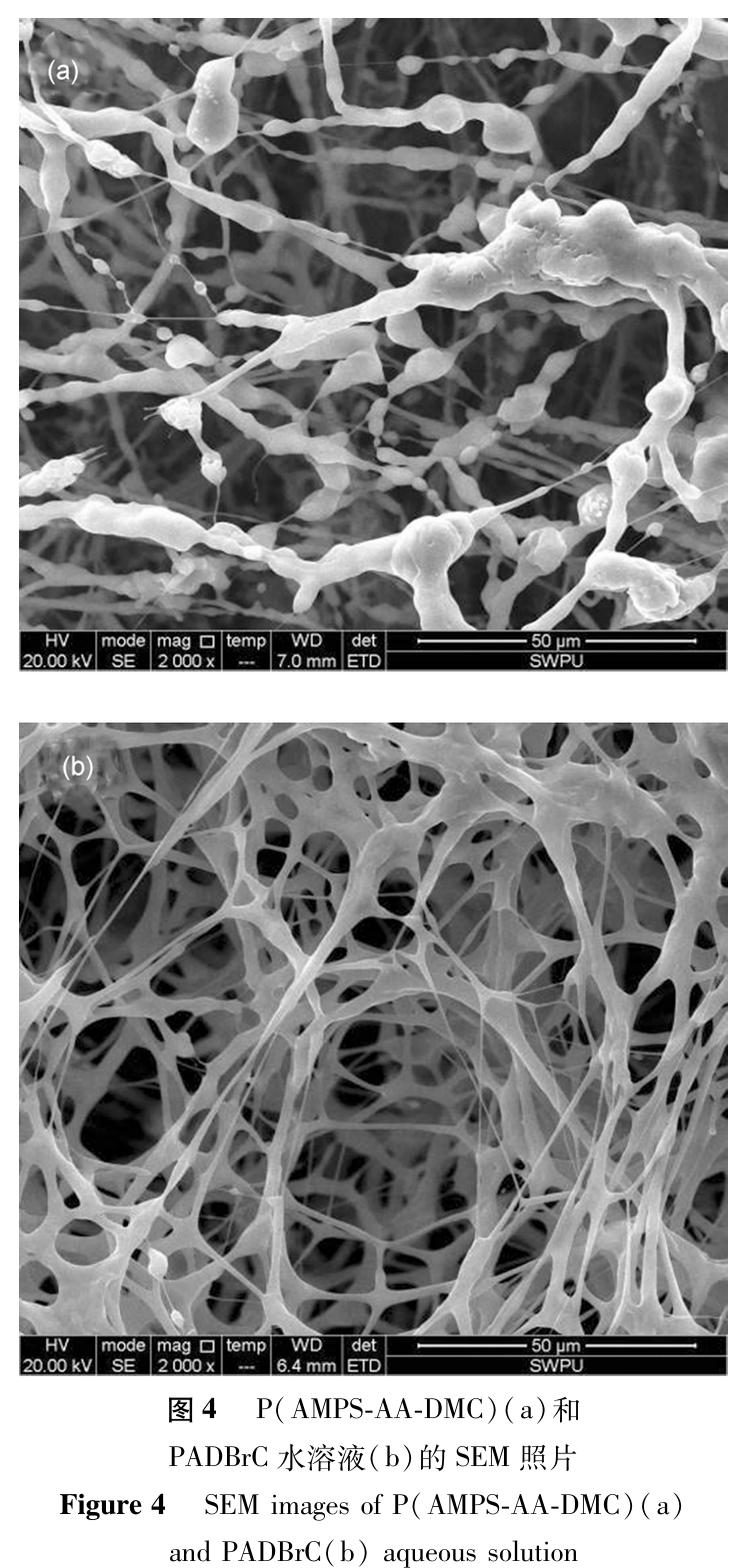
(5). The Microstructure of Polymer Aqueous Solutions
Prepare P (AMPS-AA-DMC) and PADBrC solutions with a concentration of 1.00% using distilled water, and observe the microstructure of the polymer solution using environmental scanning electron microscopy. The results are shown in Figure 4. As shown in Figure 4, there are significant differences in the microstructure between the two polymer solutions. In Figure 4 (a), P (AMPS-AA-DMC) has a chain like structure and uneven molecular chain thickness. Some parts of the same molecular chain are thicker while others are thinner. These uneven molecular chains form an irregular network structure by entanglement with each other. The reason for this phenomenon may be the introduction of cationic monomers, which have electrostatic attraction with functional groups such as -SO3H and -COOH in the molecule, causing molecular chains to fold and entangle, resulting in irregular molecular chains. In Figure 4 (b), PADBrC exhibits a network structure, which is more uniform and dense compared to P (AMPS-AA-DMC). This may be due to the introduction of DBrC into the molecule. PADBrC has long branched chains, and its steric hindrance weakens the electrostatic attraction between molecules. At the same time, due to the presence of hydrophobic side chains, the molecular chains curl and entangle, making them thicker but not forming a spherical structure.